Ch. 7 Liquids and Solids
Properties
- generally more dense than gases
- stronger attractive forces between individual particles
- liquids conform to the shape of a container and fill depending on its mass
- solids keep its shape regardless of container
Intermolecular Forces
types of forces attracting molecules to each other
Dipole-Dipole Forces
- most compounds unequally share electrons, creating a dipole
- one side partially positive (), other side partially negative ()
- need high force to overcome kinetic energy and condense to liquid
- done by increasing pressure (decrease distance) or decreasing temperature (decrease kinetic energy)
- boiling/condensation point indicates required kinetic energy
- highly polar molecules have higher boiling points than less polar molecules
London Dispersion Forces
- explanation for nonpolar gases
- electrons in constant motion create instances of instantaneous dipoles even in nonpolar substances
- can attract instantaneous dipole or induce dipole in a nearby atom/moleculex
- noble gases have weak LDFs, so have low boiling points
- polarizability: how easily an electron cloud can become a dipole
- halogens have larger electron clouds with loosely held electrons so are more polarizable higher boiling points
- in general, more electrons more polarizability
- normal alkanes: -alkane has formula , and higher have higher boiling points
- more hydrogen more instantaneous dipoles more forces and higher boiling point
Hydrogen Bonding
- large electronegativity difference between and , , create strong dipole-dipole forces called hydrogen bonds
- strongest of the three intermolecular forces
Summary
| Force | Description |
|---|---|
| LDF | weak forces caused by brief unequal electron distribution |
| Dipole-Dipole | force between partial positive and partial negative ends of molecules |
| Hydrogen Bond | strong forces between and , , or |
Physcial Properties of Liquids
- surface tension: increase in intermolecular forces at liquid surface
- when cohesive forces (attractions between identical molecules) are stronger than adhesive forces (attractions between different molecules) of a surface, the liquid beads; otherwise, uniformly spreads
- clean glass has low adhesive forces, so dishwater easily beads and evaporates, leaving spots detergent contain surfactants that reduce cohesive forces so dishwater doesn't bead
- viscosity: liquid's resistance to flow
- low attractive forces molecules move past each other easily liquid flows easily
- syrup contains sugar molecules, containing groups that hydrogen bond to water
- high temperature weaker IMFs lower viscosity
- evaporation: liquid gas
- needs sufficient kinetic energy (proportional to Kelvin temperature) to overcome IMFs
- more surface area faster evaporation (more are close to surface)
- higher temperature faster evaporation (more have kinetic energy higher than escape energy)
- liquid evaporates at a uniform rate
- at boiling point, molecules do not have to be at surface (bubbles rise to surface)
- vapor pressure: pressure of gas formed from liquid in a closed container
- in closed container, liquid evaporates, gas hits walls and liquid, condenses when hitting liquid, eventually evaporation rate condensation rate dynamic equilibrium
- dependent only on nature of liquid (attractive forces) and temperature (kinetic energy)
- natural boiling point: boiling point at ()
- higher pressure higher boiling point
- at high altitudes (low pressure environments), pressure cookers are used to increase pressure, increase boiling point, cooking food faster
- heat of vaporization (): energy needed to convert liquid into gas at normal boiling point
- symbol:
- alternatively, if is vaporized, it is called molar heat of vaporization with units
- if similar-sized molecules, hydrogen-bonded have largest , , etc.
Solids
have rigid crystal structure
- metallic crystals: rigid structure of metal nuclei and inner electrons
- very mobile valence electrons
- electric charge and thermal energy conductivity
- bonding metal atoms together varying boiling points
- energy needed often called lattice energy
- some soft, some hard/brittle, most malleable, caused by crystal structure
- substitutional alloys replace similarly-sized atoms, resulting in properties between those of the two metals
- interstitial alloys incorporate smaller atoms within existing structure with little volume change, resulting in stronger forces and stronger and harder alloy
- very mobile valence electrons
- ionic crystals: rigid crystalline structures (lattices) made of ionic compounds
- high lattice energy required to separate high melting/boiling points
- extremely brittle, as disruptive force moves like-charged ions together, causing reuplsion
- molecular crystals: crystals composed of nonmetals or covalent molecules
- held together by IMFs, weaker than ionic bonds generally soft with low melting points
- network covalent crystals: lattice structure where atoms are covalently bonded
- forms one large molecule all with covalent bonds
- can be very hard (diamond, )
- amorphous substances: noncrystalline materials
- no distinct, sharp melting point; instead gradually softening
Phase Changes
- in constant pressure, effect of heat can be explained with a heating/cooling curve
- as heat is added at a constant rate:
- solid temperature increases until melting point
- temperature stays constant until fully converted to liquid
- liquid temperature increases until boiling point
- temperature stays constant until fully converted into gas
- gas temperature increases
- opposite in a cooling curve
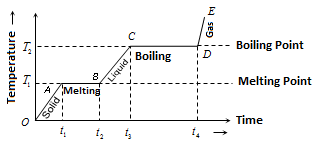
- heat capacity (): reciprocal of slope
- specific heat (): heat capacity divided by mass of sample
- molar heat/enthalpy of fusion (melting) (): first plateau, heat added divided by number of moles
- molar heat/enthalpy of vaporization (boiling) (): second plateau, head added divided by number of moles
- supercooling (in cooling curve): liquid is cooled below melting point and remaind liquid
- metastable; will rapidly crystallize if disturbed or if a seed crystal is added